Naturally Occurring Isotopes Of Iodine
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight A r°(I) |
| |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactivity except 127I, which is stable. Iodine is thus a monoisotopic element.
Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 meg years, which is far too short for it to exist equally a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect diminutive weight measurements; iodine is thus also a mononuclidic chemical element—ane that is establish in nature merely every bit a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early on nuclear tests and nuclear fission accidents.
All other iodine radioisotopes have one-half-lives less than sixty days, and 4 of these are used every bit tracers and therapeutic agents in medicine. These are 123I, 124I, 125I, and 131I. All industrial production of radioactive iodine isotopes involves these four useful radionuclides.
The isotope 135I has a half-life less than seven hours, which is too brusk to exist used in biology. Unavoidable in situ production of this isotope is important in nuclear reactor control, equally it decays to 135Xe, the virtually powerful known neutron absorber, and the nuclide responsible for the so-called iodine pit phenomenon.
In addition to commercial production, 131I (half-life 8 days) is one of the common radioactive fission products of nuclear fission, and is thus produced inadvertently in very big amounts within nuclear reactors. Due to its volatility, brusk half-life, and high abundance in fission products, 131I (along with the short-lived iodine isotope 132I, which is produced from the decay of 132Te with a half-life of iii days) is responsible for the largest role of radioactive contamination during the get-go calendar week later on adventitious environmental contamination from the radioactive waste from a nuclear power plant. Thus highly dosed iodine supplements (ordinarily potassium iodide) are given to the populace after nuclear accidents or explosions (and in some cases prior to any such incident as a civil defense mechanism) to reduce the uptake of radioactive iodine compounds by the thyroid before the highly radioactive isotopes have had time to decay.
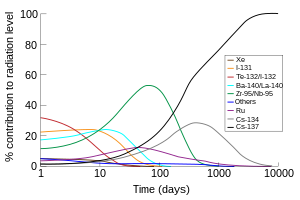
The portion of the total radiation activeness (in air) contributed by each isotope versus time after the Chernobyl disaster, at the site. Note the prominence of radiation from I-131 and Te-132/I-132 for the commencement week. (Prototype using data from the OECD written report, and the second edition of 'The radiochemical manual'.[3])
List of isotopes [edit]
| Nuclide [n ane] | Z | Northward | Isotopic mass (Da) [n two] [n iii] | One-half-life [n 4] | Decay mode [due north v] | Daughter isotope [northward 6] [n 7] | Spin and parity [n 8] [n iv] | Natural abundance (mole fraction) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy[north iv] | Normal proportion | Range of variation | |||||||||||||||||
| 108I | 53 | 55 | 107.94348(39)# | 36(6) ms | α (xc%) | 104Sb | (1)# | ||||||||||||
| β+ (9%) | 108Te | ||||||||||||||||||
| p (1%) | 107Te | ||||||||||||||||||
| 109I | 53 | 56 | 108.93815(11) | 103(5) µs | p (99.five%) | 108Te | (5/2+) | ||||||||||||
| α (.5%) | 105Sb | ||||||||||||||||||
| 110I | 53 | 57 | 109.93524(33)# | 650(twenty) ms | β+ (lxx.9%) | 110Te | 1+# | ||||||||||||
| α (17%) | 106Sb | ||||||||||||||||||
| β+, p (11%) | 109Sb | ||||||||||||||||||
| β+, α (one.09%) | 106Sn | ||||||||||||||||||
| 111I | 53 | 58 | 110.93028(32)# | ii.5(2) south | β+ (99.92%) | 111Te | (five/2+)# | ||||||||||||
| α (.088%) | 107Sb | ||||||||||||||||||
| 112I | 53 | 59 | 111.92797(23)# | 3.42(11) south | β+ (99.01%) | 112Te | |||||||||||||
| β+, p (.88%) | 111Sb | ||||||||||||||||||
| β+, α (.104%) | 108Sn | ||||||||||||||||||
| α (.0012%) | 108Sb | ||||||||||||||||||
| 113I | 53 | lx | 112.92364(6) | half-dozen.6(2) s | β+ (100%) | 113Te | 5/2+# | ||||||||||||
| α (3.three×10−7%) | 109Sb | ||||||||||||||||||
| β+, α | 109Sn | ||||||||||||||||||
| 114I | 53 | 61 | 113.92185(32)# | 2.one(ii) s | β+ | 114Te | 1+ | ||||||||||||
| β+, p (rare) | 113Sb | ||||||||||||||||||
| 114mI | 265.9(five) keV | half dozen.2(5) s | β+ (91%) | 114Te | (7) | ||||||||||||||
| IT (9%) | 114I | ||||||||||||||||||
| 115I | 53 | 62 | 114.91805(3) | 1.3(two) min | β+ | 115Te | (5/2+)# | ||||||||||||
| 116I | 53 | 63 | 115.91681(10) | 2.91(15) southward | β+ | 116Te | one+ | ||||||||||||
| 116mI | 400(50)# keV | 3.27(16) µs | (7−) | ||||||||||||||||
| 117I | 53 | 64 | 116.91365(3) | ii.22(4) min | β+ | 117Te | (five/two)+ | ||||||||||||
| 118I | 53 | 65 | 117.913074(21) | 13.vii(5) min | β+ | 118Te | 2− | ||||||||||||
| 118mI | 190.one(10) keV | 8.5(5) min | β+ | 118Te | (vii−) | ||||||||||||||
| IT (rare) | 118I | ||||||||||||||||||
| 119I | 53 | 66 | 118.91007(iii) | 19.ane(4) min | β+ | 119Te | 5/2+ | ||||||||||||
| 120I | 53 | 67 | 119.910048(nineteen) | 81.half dozen(2) min | β+ | 120Te | 2− | ||||||||||||
| 120m1I | 72.61(9) keV | 228(15) ns | (1+, ii+, three+) | ||||||||||||||||
| 120m2I | 320(15) keV | 53(four) min | β+ | 120Te | (7−) | ||||||||||||||
| 121I | 53 | 68 | 120.907367(11) | 2.12(1) h | β+ | 121Te | 5/2+ | ||||||||||||
| 121mI | 2376.ix(4) keV | ix.0(15) µs | |||||||||||||||||
| 122I | 53 | 69 | 121.907589(6) | 3.63(6) min | β+ | 122Te | 1+ | ||||||||||||
| 123I[n 9] | 53 | 70 | 122.905589(4) | thirteen.2235(nineteen) h | EC | 123Te | 5/2+ | ||||||||||||
| 124I[north 9] | 53 | 71 | 123.9062099(25) | 4.1760(3) d | β+ | 124Te | ii− | ||||||||||||
| 125I[n 9] | 53 | 72 | 124.9046302(xvi) | 59.400(10) d | EC | 125Te | 5/2+ | ||||||||||||
| 126I | 53 | 73 | 125.905624(iv) | 12.93(5) d | β+ (56.3%) | 126Te | 2− | ||||||||||||
| β− (43.7%) | 126Xe | ||||||||||||||||||
| 127I[n 10] | 53 | 74 | 126.904473(four) | Stable [n 11] | five/2+ | 1.0000 | |||||||||||||
| 128I | 53 | 75 | 127.905809(4) | 24.99(2) min | β− (93.ane%) | 128Xe | ane+ | ||||||||||||
| β+ (six.9%) | 128Te | ||||||||||||||||||
| 128m1I | 137.850(iv) keV | 845(20) ns | four− | ||||||||||||||||
| 128m2I | 167.367(5) keV | 175(15) ns | (6)− | ||||||||||||||||
| 129I[north ten] [n 12] | 53 | 76 | 128.904988(iii) | 1.57(four)×107 y | β− | 129Xe | 7/2+ | Trace[northward thirteen] | |||||||||||
| 130I | 53 | 77 | 129.906674(iii) | 12.36(one) h | β− | 130Xe | 5+ | ||||||||||||
| 130m1I | 39.9525(13) keV | viii.84(6) min | It (84%) | 130I | 2+ | ||||||||||||||
| β− (xvi%) | 130Xe | ||||||||||||||||||
| 130m2I | 69.5865(7) keV | 133(seven) ns | (vi)− | ||||||||||||||||
| 130m3I | 82.3960(19) keV | 315(xv) ns | - | ||||||||||||||||
| 130m4I | 85.1099(10) keV | 254(4) ns | (half dozen)− | ||||||||||||||||
| 131I[n 10] [north 9] | 53 | 78 | 130.9061246(12) | 8.02070(xi) d | β− | 131Xe | 7/2+ | ||||||||||||
| 132I | 53 | 79 | 131.907997(6) | two.295(13) h | β− | 132Xe | 4+ | ||||||||||||
| 132mI | 104(12) keV | ane.387(fifteen) h | IT (86%) | 132I | (8−) | ||||||||||||||
| β− (14%) | 132Xe | ||||||||||||||||||
| 133I | 53 | lxxx | 132.907797(5) | 20.viii(1) h | β− | 133Xe | seven/2+ | ||||||||||||
| 133m1I | 1634.174(17) keV | 9(two) s | Information technology | 133I | (xix/two−) | ||||||||||||||
| 133m2I | 1729.160(17) keV | ~170 ns | (15/2−) | ||||||||||||||||
| 134I | 53 | 81 | 133.909744(9) | 52.5(2) min | β− | 134Xe | (4)+ | ||||||||||||
| 134mI | 316.49(22) keV | 3.52(4) min | Information technology (97.7%) | 134I | (8)− | ||||||||||||||
| β− (2.3%) | 134Xe | ||||||||||||||||||
| 135I[n 14] | 53 | 82 | 134.910048(8) | 6.57(ii) h | β− | 135Xe | seven/2+ | ||||||||||||
| 136I | 53 | 83 | 135.91465(5) | 83.4(10) due south | β− | 136Xe | (1−) | ||||||||||||
| 136mI | 650(120) keV | 46.ix(x) s | β− | 136Xe | (6−) | ||||||||||||||
| 137I | 53 | 84 | 136.917871(30) | 24.13(12) s | β− (92.86%) | 137Xe | (vii/ii+) | ||||||||||||
| β−, n (7.14%) | 136Xe | ||||||||||||||||||
| 138I | 53 | 85 | 137.92235(9) | six.23(3) south | β− (94.54%) | 138Xe | (2−) | ||||||||||||
| β−, due north (5.46%) | 137Xe | ||||||||||||||||||
| 139I | 53 | 86 | 138.92610(3) | 2.282(10) s | β− (90%) | 139Xe | 7/ii+# | ||||||||||||
| β−, northward (10%) | 138Xe | ||||||||||||||||||
| 140I | 53 | 87 | 139.93100(21)# | 860(40) ms | β− (xc.vii%) | 140Xe | (three)(−#) | ||||||||||||
| β−, n (ix.3%) | 139Xe | ||||||||||||||||||
| 141I | 53 | 88 | 140.93503(21)# | 430(20) ms | β− (78%) | 141Xe | seven/ii+# | ||||||||||||
| β−, due north (22%) | 140Xe | ||||||||||||||||||
| 142I | 53 | 89 | 141.94018(43)# | ~200 ms | β− (75%) | 142Xe | 2−# | ||||||||||||
| β−, n (25%) | 141Xe | ||||||||||||||||||
| 143I | 53 | 90 | 142.94456(43)# | 100# ms [> 300 ns] | β− | 143Xe | 7/2+# | ||||||||||||
| 144I | 53 | 91 | 143.94999(54)# | 50# ms [> 300 ns] | β− | 144Xe | i−# | ||||||||||||
| This tabular array header & footer: | |||||||||||||||||||
- ^ thousandI – Excited nuclear isomer.
- ^ ( ) – Incertitude (1σ) is given in concise form in parentheses after the respective last digits.
- ^ # – Diminutive mass marked #: value and incertitude derived not from purely experimental data, simply at least partly from trends from the Mass Surface (TMS).
- ^ a b c # – Values marked # are not purely derived from experimental data, just at least partly from trends of neighboring nuclides (TNN).
- ^ Modes of decay:
- ^ Bold italics symbol as daughter – Daughter product is almost stable.
- ^ Bold symbol as girl – Daughter product is stable.
- ^ ( ) spin value – Indicates spin with weak consignment arguments.
- ^ a b c d Has medical uses
- ^ a b c Fission product
- ^ Theoretically capable of spontaneous fission
- ^ Can be used to date sure early events in Solar System history and some employ for dating groundwater
- ^ Cosmogenic nuclide, too found as nuclear contamination
- ^ Produced equally a decay product of 135Te in nuclear reactors, in turn decays to 135Xe, which, if allowed to build upwards, can shut downwardly reactors due to the iodine pit phenomenon
Notable radioisotopes [edit]
Radioisotopes of iodine are called radioactive iodine or radioiodine. Dozens exist, but about a half dozen are the most notable in practical sciences such as the life sciences and nuclear ability, as detailed below. Mentions of radioiodine in health care contexts refer more than often to iodine-131 than to other isotopes.
Of the many isotopes of iodine, but 2 are typically used in a medical setting: iodine-123 and iodine-131. Since 131I has both a beta and gamma disuse mode, it can be used for radiotherapy or for imaging. 123I, which has no beta activity, is more suited for routine nuclear medicine imaging of the thyroid and other medical processes and less damaging internally to the patient. In that location are some situations in which iodine-124 and iodine-125 are too used in medicine.[4]
Due to preferential uptake of iodine past the thyroid, radioiodine is extensively used in imaging of and, in the case of 131I, destroying dysfunctional thyroid tissues. Other types of tissue selectively have up certain iodine-131-containing tissue-targeting and killing radiopharmaceutical agents (such as MIBG). Iodine-125 is the simply other iodine radioisotope used in radiation therapy, merely simply as an implanted capsule in brachytherapy, where the isotope never has a chance to be released for chemical interaction with the body's tissues.
Iodine-123 and iodine-125 [edit]
The gamma-emitting isotopes iodine-123 (half-life thirteen hours), and (less normally) the longer-lived and less energetic iodine-125 (half-life 59 days) are used as nuclear imaging tracers to evaluate the anatomic and physiologic office of the thyroid. Abnormal results may exist caused by disorders such as Graves' disease or Hashimoto'due south thyroiditis. Both isotopes decay by electron capture (EC) to the corresponding tellurium nuclides, but in neither case are these the metastable nuclides 123mTe and 125mTe (which are of higher free energy, and are not produced from radioiodine). Instead, the excited tellurium nuclides decay immediately (half-life too short to detect). Following EC, the excited 123Te from 123I emits a loftier-speed 127 keV internal conversion electron (non a beta ray) about 13% of the time, but this does little cellular damage due to the nuclide'south short one-half-life and the relatively pocket-sized fraction of such events. In the residual of cases, a 159 keV gamma ray is emitted, which is well-suited for gamma imaging.
Excited 125Te resulting from electron capture of 125I also emits a much lower-energy internal conversion electron (35.5 keV), which does relatively niggling damage due to its low energy, even though its emission is more common. The relatively low-energy gamma from 125I/125Te decay is poorly suited for imaging, only can still be seen, and this longer-lived isotope is necessary in tests that require several days of imaging, for instance, fibrinogen scan imaging to detect blood clots.
Both 123I and 125I emit copious depression energy Auger electrons after their disuse, but these practise not cause serious damage (double-stranded DNA breaks) in cells, unless the nuclide is incorporated into a medication that accumulates in the nucleus, or into DNA (this is never the case is clinical medicine, but it has been seen in experimental animal models).[5]
Iodine-125 is also unremarkably used by radiation oncologists in low dose rate brachytherapy in the treatment of cancer at sites other than the thyroid, peculiarly in prostate cancer. When 125I is used therapeutically, it is encapsulated in titanium seeds and implanted in the area of the tumor, where it remains. The low energy of the gamma spectrum in this example limits radiation damage to tissues far from the implanted capsule. Iodine-125, due to its suitable longer half-life and less penetrating gamma spectrum, is too often preferred for laboratory tests that rely on iodine every bit a tracer that is counted by a gamma counter, such as in radioimmunoassaying.
125I is used as the radiolabel in investigating which ligands go to which plant pattern recognition receptors (PRRs).[vi]
Iodine-124 [edit]
Iodine-124 is a proton-rich isotope of iodine with a one-half-life of four.18 days. Its modes of disuse are: 74.4% electron capture, 25.6% positron emission. 124I decays to 124Te. Iodine-124 can exist fabricated by numerous nuclear reactions via a cyclotron. The nigh common starting material used is 124Te.
Iodine-124 as the iodide common salt tin can be used to directly image the thyroid using positron emission tomography (PET).[7] Iodine-124 can also be used equally a PET radiotracer with a usefully longer half-life compared with fluorine-xviii.[8] In this use, the nuclide is chemically bonded to a pharmaceutical to form a positron-emitting radiopharmaceutical, and injected into the trunk, where once again information technology is imaged by PET scan.
Iodine-129 [edit]
Iodine-129 (129I; half-life xv.7 million years) is a product of cosmic ray spallation on various isotopes of xenon in the temper, in cosmic ray muon interaction with tellurium-130, and also uranium and plutonium fission, both in subsurface rocks and nuclear reactors. Artificial nuclear processes, in particular nuclear fuel reprocessing and atmospheric nuclear weapons tests, have now swamped the natural signal for this isotope. Even so, it now serves as a groundwater tracer as indicator of nuclear waste dispersion into the natural environment. In a like style, 129I was used in rainwater studies to track fission products post-obit the Chernobyl disaster.
In some ways, 129I is similar to 36Cl. It is a soluble halogen, exists mainly equally a not-sorbing anion, and is produced by cosmogenic, thermonuclear, and in-situ reactions. In hydrologic studies, 129I concentrations are normally reported as the ratio of 129I to total I (which is nigh all 127I). As is the example with 36Cl/Cl, 129I/I ratios in nature are quite small, 10−14 to x−10 (peak thermonuclear 129I/I during the 1960s and 1970s reached about 10−7). 129I differs from 36Cl in that its half-life is longer (15.7 vs. 0.301 million years), information technology is highly biophilic, and occurs in multiple ionic forms (commonly, I− and IO3 −), which have different chemical behaviors. This makes it fairly easy for 129I to enter the biosphere as information technology becomes incorporated into vegetation, soil, milk, animal tissue, etc. Excesses of stable 129Xe in meteorites have been shown to result from decay of "primordial" iodine-129 produced newly by the supernovas that created the dust and gas from which the solar system formed. This isotope has long decayed and is thus referred to equally "extinct". Historically, 129I was the first extinct radionuclide to exist identified every bit nowadays in the early Solar System. Its decay is the basis of the I-Xe iodine-xenon radiometric dating scheme, which covers the first 85 meg years of Solar System development.
Iodine-131 [edit]

A Pheochromocytoma is seen every bit a nighttime sphere in the center of the trunk (information technology is in the left adrenal gland). Paradigm is by MIBG scintigraphy, with radiation from radioiodine in the MIBG. 2 images are seen of the same patient from front end and back. Notation the dark image of the thyroid due to unwanted uptake of radioiodine from the medication past the thyroid gland in the neck. Accumulation at the sides of the head is from salivary gland uptake of iodide. Radioactivity is also seen in the bladder.
Iodine-131 ( 131
I
) is a beta-emitting isotope with a one-half-life of eight days, and insufficiently energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.vi to 2.0 mm from the site of uptake. This beta radiation can exist used for the destruction of thyroid nodules or hyperfunctioning thyroid tissue and for elimination of remaining thyroid tissue after surgery for the treatment of Graves' disease. The purpose of this therapy, which was first explored by Dr. Saul Hertz in 1941,[9] is to destroy thyroid tissue that could non exist removed surgically. In this procedure, 131I is administered either intravenously or orally following a diagnostic browse. This procedure may also be used, with higher doses of radio-iodine, to treat patients with thyroid cancer.
The 131I is taken upwardly into thyroid tissue and concentrated there. The beta particles emitted by the radioisotope destroys the associated thyroid tissue with little damage to surrounding tissues (more than 2.0 mm from the tissues absorbing the iodine). Due to similar destruction, 131I is the iodine radioisotope used in other water-soluble iodine-labeled radiopharmaceuticals (such as MIBG) used therapeutically to destroy tissues.
The high energy beta radiation (upward to 606 keV) from 131I causes information technology to be the almost carcinogenic of the iodine isotopes. It is thought to cause the majority of backlog thyroid cancers seen later on nuclear fission contagion (such as bomb fallout or severe nuclear reactor accidents like the Chernobyl disaster) However, these epidemiological effects are seen primarily in children, and handling of adults and children with therapeutic 131I, and epidemiology of adults exposed to low-dose 131I has not demonstrated carcinogenicity.[10]
Iodine-135 [edit]
Iodine-135 is an isotope of iodine with a half-life of 6.6 hours. It is an important isotope from the viewpoint of nuclear reactor physics. Information technology is produced in relatively large amounts as a fission production, and decays to xenon-135, which is a nuclear toxicant with a very large thermal neutron cantankerous section, which is a crusade of multiple complications in the control of nuclear reactors. The process of buildup of xenon-135 from accumulated iodine-135 tin temporarily foreclose a shut-down reactor from restarting. This is known as xenon poisoning or "falling into an iodine pit".
Iodine-128 and other isotopes [edit]
Iodine fission-produced isotopes not discussed to a higher place (iodine-128, iodine-130, iodine-132, and iodine-133) take half-lives of several hours or minutes, rendering them almost useless in other applicable areas. Those mentioned are neutron-rich and undergo beta disuse to isotopes of xenon. Iodine-128 (half-life 25 minutes) tin disuse to either tellurium-128 past electron capture or to xenon-128 past beta decay. It has a specific radioactivity of two.177×106 TBq/g.
Nonradioactive iodide (127I) as protection from unwanted radioiodine uptake by the thyroid [edit]
Colloquially, radioactive materials tin can be described equally "hot," and non-radioactive materials can be described as "cold." There are instances in which cold iodide is administered to people in order to foreclose the uptake of hot iodide by the thyroid gland. For example, blockade of thyroid iodine uptake with potassium iodide is used in nuclear medicine scintigraphy and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane (MIBG), which is used to image or treat neural tissue tumors, or iodinated fibrinogen, which is used in fibrinogen scans to investigate clotting. These compounds comprise iodine, but not in the iodide form. However, since they may be ultimately metabolized or break downwardly to radioactive iodide, it is mutual to administrate not-radioactive potassium iodide to insure that metabolites of these radiopharmaceuticals is not sequestered by thyroid gland and inadvertently administrate a radiological dose to that tissue.
Potassium iodide has been distributed to populations exposed to nuclear fission accidents such as the Chernobyl disaster. The iodide solution SSKI, a saturated due southolution of potassium (K) iodide in water, has been used to block absorption of the radioiodine (it has no result on other radioisotopes from fission). Tablets containing potassium iodide are now as well manufactured and stocked in fundamental disaster sites by some governments for this purpose. In theory, many harmful late-cancer effects of nuclear fallout might exist prevented in this way, since an excess of thyroid cancers, presumably due to radioiodine uptake, is the only proven radioisotope contamination effect after a fission accident, or from contagion by fallout from an diminutive bomb (prompt radiations from the bomb also causes other cancers, such as leukemias, direct). Taking large amounts of iodide saturates thyroid receptors and prevents uptake of most radioactive iodine-131 that may be present from fission product exposure (although it does not protect from other radioisotopes, nor from any other class of direct radiation). The protective effect of KI lasts approximately 24 hours, and so must exist dosed daily until a risk of significant exposure to radioiodines from fission products no longer exists.[eleven] [12] Iodine-131 (the about mutual radioiodine contaminant in fallout) likewise decays relatively rapidly with a half-life of eight days, and so that 99.95% of the original radioiodine has vanished after 3 months.
References [edit]
- Isotope masses from:
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: three–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- Isotopic compositions and standard atomic masses from:
- de Laeter, John Robert; Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003). "Diminutive weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- Wieser, Michael E. (2006). "Atomic weights of the elements 2005 (IUPAC Technical Written report)". Pure and Applied Chemical science. 78 (11): 2051–2066. doi:ten.1351/pac200678112051.
- "News & Notices: Standard Atomic Weights Revised". International Union of Pure and Applied Chemical science. 19 October 2005.
- Half-life, spin, and isomer data selected from the post-obit sources.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay backdrop", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- National Nuclear Data Center. "NuDat 2.x database". Brookhaven National Laboratory.
- Holden, Norman E. (2004). "eleven. Tabular array of the Isotopes". In Lide, David R. (ed.). CRC Handbook of Chemistry and Physics (85th ed.). Boca Raton, Florida: CRC Press. ISBN978-0-8493-0485-9.
- ^ "Standard Atomic Weights: Iodine". CIAAW. 1985.
- ^ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ^ "Nuclear Data Evaluation Lab". Archived from the original on 2007-01-21. Retrieved 2009-05-13 .
- ^ Augustine George; James T Lane; Arlen D Meyers (January 17, 2013). "Radioactive Iodine Uptake Testing". Medscape.
- ^ Five. R. Narra; et al. (1992). "Radiotoxicity of Some Iodine-123, Iodine-125, and Iodine-131-Labeled Compounds in Mouse Testes: Implications for Radiopharmaceutical Pattern" (PDF). Journal of Nuclear Medicine. 33 (12): 2196–201. PMID 1460515.
- ^ Boutrot, Freddy; Zipfel, Cyril (2017-08-04). "Office, Discovery, and Exploitation of Plant Design Recognition Receptors for Wide-Spectrum Disease Resistance". Annual Review of Phytopathology. Annual Reviews. 55 (1): 257–286. doi:10.1146/annurev-phyto-080614-120106. ISSN 0066-4286. PMID 28617654.
- ^ E. Rault; et al. (2007). "Comparing of Image Quality of Dissimilar Iodine Isotopes (I-123, I-124, and I-131)". Cancer Biotherapy & Radiopharmaceuticals. 22 (iii): 423–430. doi:10.1089/cbr.2006.323. PMID 17651050.
- ^ BV Cyclotron VU, Amsterdam, 2016, Information on Iodine-124 for PET
- ^ Hertz, Barbara; Schuleller, Kristin (2010). "Saul Hertz, MD (1905 - 1950) A Pioneer in the Employ of Radioactive Iodine". Endocrine Practice. sixteen (iv): 713–715. doi:10.4158/EP10065.CO. PMID 20350908.
- ^ Robbins, Jacob; Schneider, Arthur B. (2000). "Thyroid cancer following exposure to radioactive iodine". Reviews in Endocrine and Metabolic Disorders. 1 (three): 197–203. doi:10.1023/A:1010031115233. ISSN 1389-9155. PMID 11705004. S2CID 13575769.
- ^ "Frequently Asked Questions on Potassium Iodide". Food and Drug Administration. Retrieved 2009-06-06 .
- ^ "Potassium Iodide as a Thyroid Blocking Amanuensis in Radiation Emergencies". Federal Register. Food and Drug Administration. Archived from the original on 2011-10-02. Retrieved 2009-06-06 .
External links [edit]
- Iodine isotopes data from The Berkeley Laboratory Isotopes Project'due south
- Iodine-128, Iodine-130, Iodine-132 information from 'Wolframalpha'
Naturally Occurring Isotopes Of Iodine,
Source: https://en.wikipedia.org/wiki/Isotopes_of_iodine
Posted by: stewartafre1969.blogspot.com


0 Response to "Naturally Occurring Isotopes Of Iodine"
Post a Comment